Master of Science Program
From the Operating Room to Innovation—Launch Your Biomedical Career Here.
A customer-focused product development curriculum
At the Center for Medical Technology & Innovation (CMTI), you won’t just study biomedical engineering—you’ll live it. From your very first week, you’ll step into the operating room, work directly with surgeons, and begin turning unmet clinical needs into real medical device solutions.
Located only 981 steps from the University of Rochester Medical Center, our Department of Biomedical Engineering offers unmatched access to clinicians and procedures. Whether observing scheduled surgeries or responding to emergent cases, you’ll be right where innovation happens—without the hassle of long commutes.
Our curriculum is built around the full product development process, giving you the skills that matter most to industry. You’ll dive into:
Medical Device Design – from concept to prototype
Quality Systems & FDA Regulations – learn how safe, effective devices reach patients
Intellectual Property Strategy – understand how to protect and translate innovation
Human Anatomy for Engineers – gain the clinical context behind your designs
This unique blend of clinical immersion, hands-on design, and industry-focused coursework ensures you graduate ready to make an immediate impact.

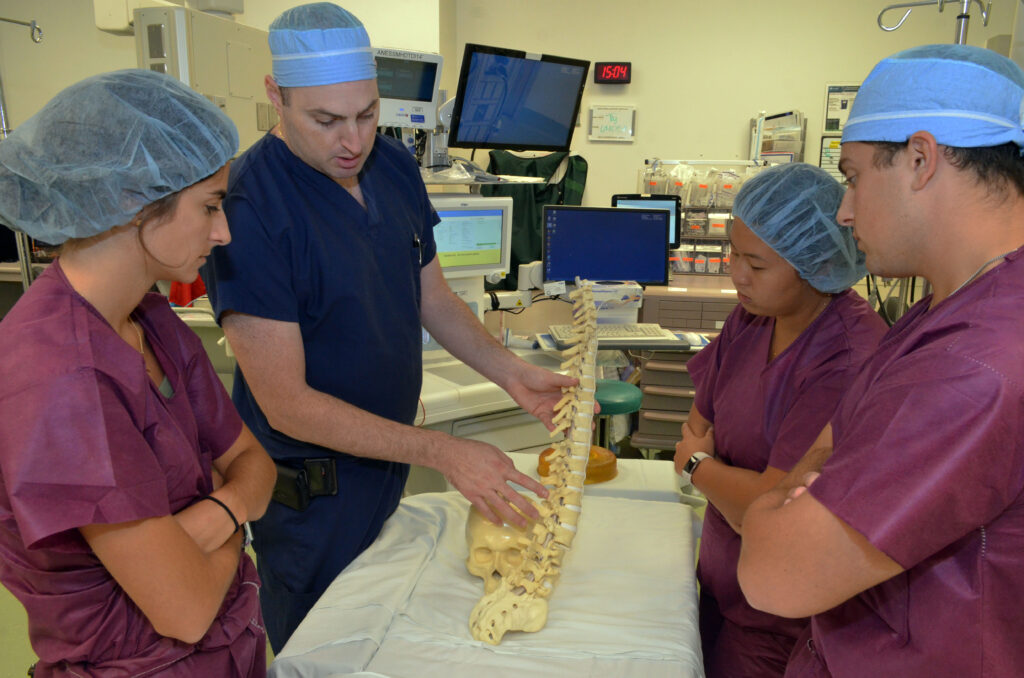
Being in the OR is no small feat! With CMTI’s training and our unique proximity to the University of Rochester Medical Center, students log 130+ hours collaborating with surgeons at the front line of innovation.
Why Choose the CMTI?
Experience That Sets You Apart
Most Master’s programs prepare you for industry. At CMTI, we take it a step further: we prepare you to lead.
Here’s what makes us unique:
Clinical Immersion: Observe surgeries and shadow clinicians in real hospital settings.
Surgeon Collaboration: Partner directly with leading surgeons throughout the design process.
Hands-On Prototyping: Translate clinical insights into functional device prototypes.
Regulatory & IP Training: Learn the essentials of medical device approval, reimbursement, and intellectual property.
Career Advantage: Build skills typically reserved for senior engineers—before you even graduate.
The CMTI Experience
A Year of Discovery, Design, and Impact
Our program is structured to mirror the real-world innovation pipeline:
Summer: Clinical Rotations — Join a clinical specialty, observe surgeries, and capture dozens of unmet clinical needs.
Fall Semester: Needs Filtering & Concept Generation — Narrow to the most promising opportunities, brainstorm solutions, and begin prototyping.
Spring Semester: Design Refinement & Translation — Advance prototypes, evaluate risk, and explore IP, regulatory, and reimbursement strategies.
By graduation, you won’t just have a degree—you’ll have a portfolio of real-world innovations and the experience to match.
Student Voices
What Our Students Say
“You don’t just learn about device development—you live it.”
“The OR rotations gave me perspective I could never get from a textbook.”
“Because of CMTI, I entered my first job already fluent in surgeon collaboration and design translation.”
“CMTI gave me the unique opportunity to work with surgeons from day one. That experience made me stand out to employers and accelerated my career path.”
Prospective Students
We are incredibly proud of the success of our Alumni for their continuous contributions to Medicine and Engineering.
Their career paths offer an excellent view of the diverse positions that are available and how a student with a MS degree can navigate the Medical Technology and Innovation industry.
Discover how CMTI can prepare you for your future career in MedTech! Connect with us today and learn about our MS application process.
Meliora!