CMTI Students Sweep the Competition at the First Annual SMD-AIN Big Pitch!
Innovative Medical Device Concepts Propel Teams to the Top

STMedical
The triumphant trio - Stephen Wells, Hadi Wehbe, and Lucas Lassinger - claimed first place at the AIN Center competition, showcasing their brilliance as CMTI Master's students. Their game-changing STMedical solution, a safe trocar insertion method, wowed the judges and secured them a well-deserved $500 prize!
ORIF Screw
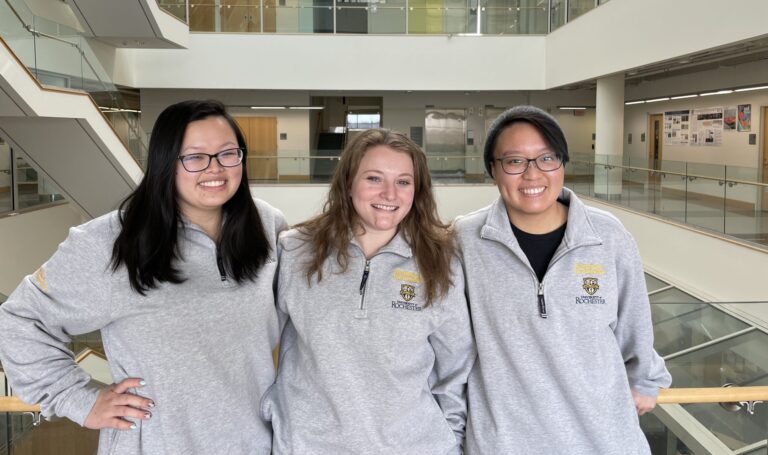
Kudos to the talented CMTI Master's students - Abbi Miller, Allie Coon, and Sylvia Zhong - for bagging second place! Their innovative ORIF device captivated the judges, earning them a fantastic $250 reward. We foresee these rising stars making a significant impact in the medical technology sphere and eagerly anticipate their future accomplishments.
Congratulations to all teams who participated and to the CMTI students who took home First and Second place!
Discover these now CMTI Alumni's successful career paths!

Lucas Lassinger
Product Manageer - Med Dimensions

Hadi Wehbe
R&D Engineer - Casana

Steve Wells
Process Validation Engineer - LSI Solutions

Allie Coon
Quality Engineer - Baxter International

Abbi Miller
Process Engineer - Janssen Pharmaceuticals R&D









