Program Requirements
M.S. in Biomedical Engineering Degree Details
Take part in University of Rochester’s CMTI one-year MS degree program, designed to equip students with distinctive experience in clinical observation and bioengineering design.
This program is part of the Biomedical Engineering Plan B coursework structure. Students in the program must fulfill a minimum of 30 credit hours during the academic year in addition to various presentations like clinical needs reviews, design reviews, human factors presentations, product feasibility presentations, and more!
These aspects are not merely academic requirements but are integral experiences that pave the way to a dynamic and fulfilling career in biomedical engineering. Students will find themselves at the cutting edge of technology and innovation, fully equipped with the skills and knowledge needed to excel in the field.
Download Here
CMTI Academic Schedule PDF

Medical Device Design
Biomedical Engineering MS Program



Core Curriculum (18 credits)
BME 498 (tuition free!)
Course Title – CMTI Summer Rotation
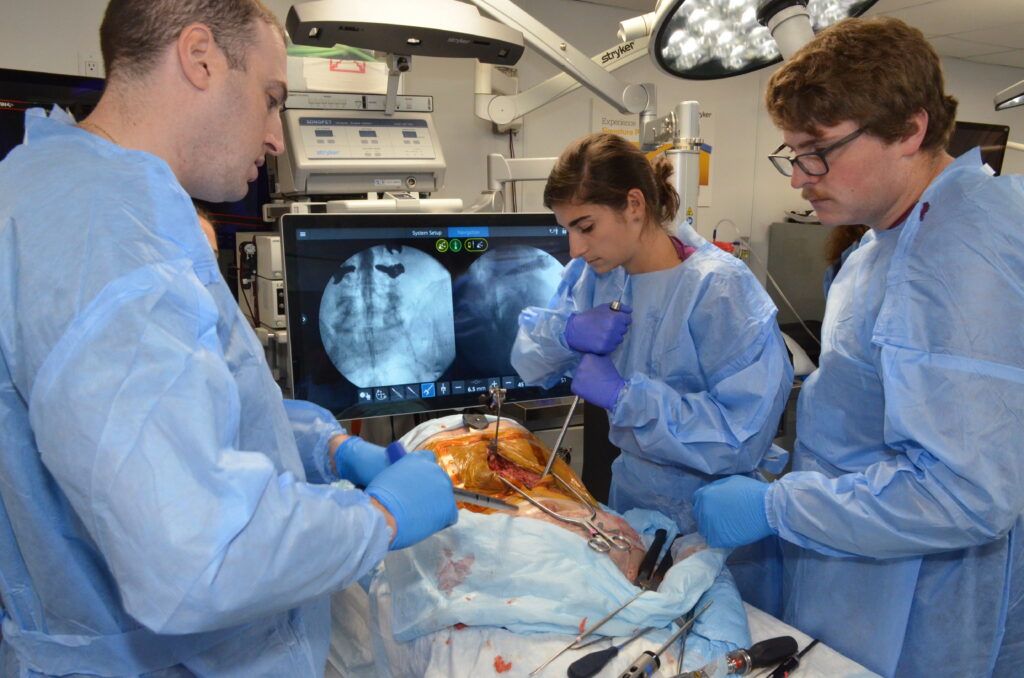
- 8-week Clinical Practicum in the Medical Device Design MS program
- Starting early July for incoming students
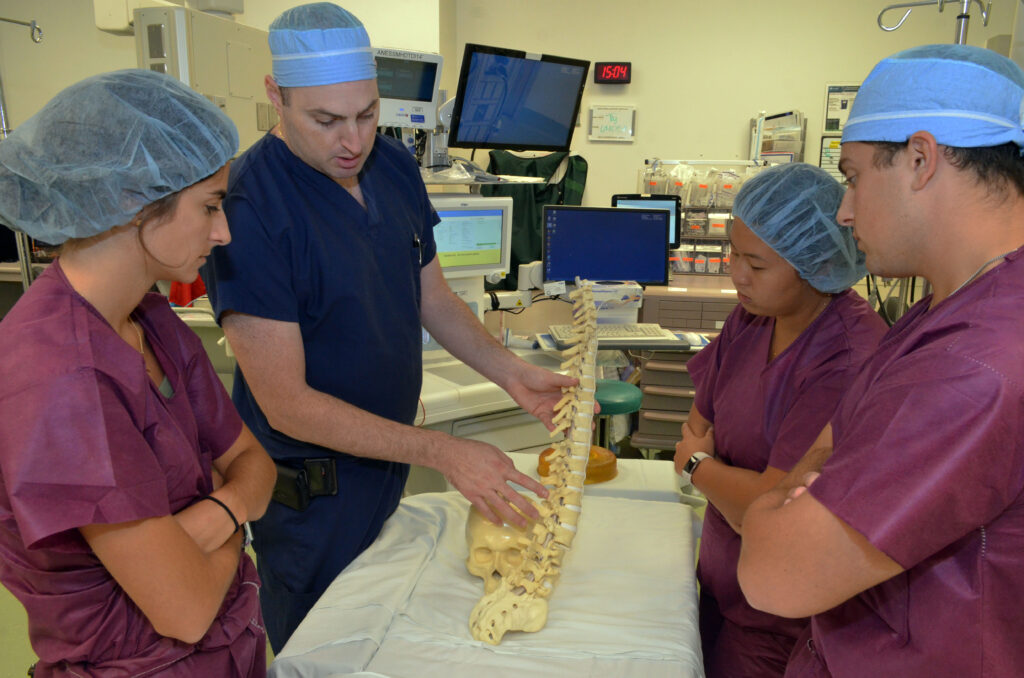
- Observation of varied clinical teams, surgeries, and specialties (orthopedic, cardiac surgery, neurosurgery, etc.)
- Guided needs finding, foundation for all design activities throughout the year
- Interaction with residents, attending surgeons, nursing staff, and company representatives
- Understand pains and gains of potential clinical customers
- Develop skills to identify unmet clinical needs in the operating room
- Combine engineering background with the life-saving work of the clinical space
- Cultivate talents, make a difference in people’s lives, and empower next-generation biomedical engineers
- Opportunity to collaborate with clinicians, shadow surgeries, create innovative solutions, and improve patient outcomes
- Shadow at one of the top research facilities in New York
- Connect with medical professionals and gain deeper understanding of the medical technology field
BME 535
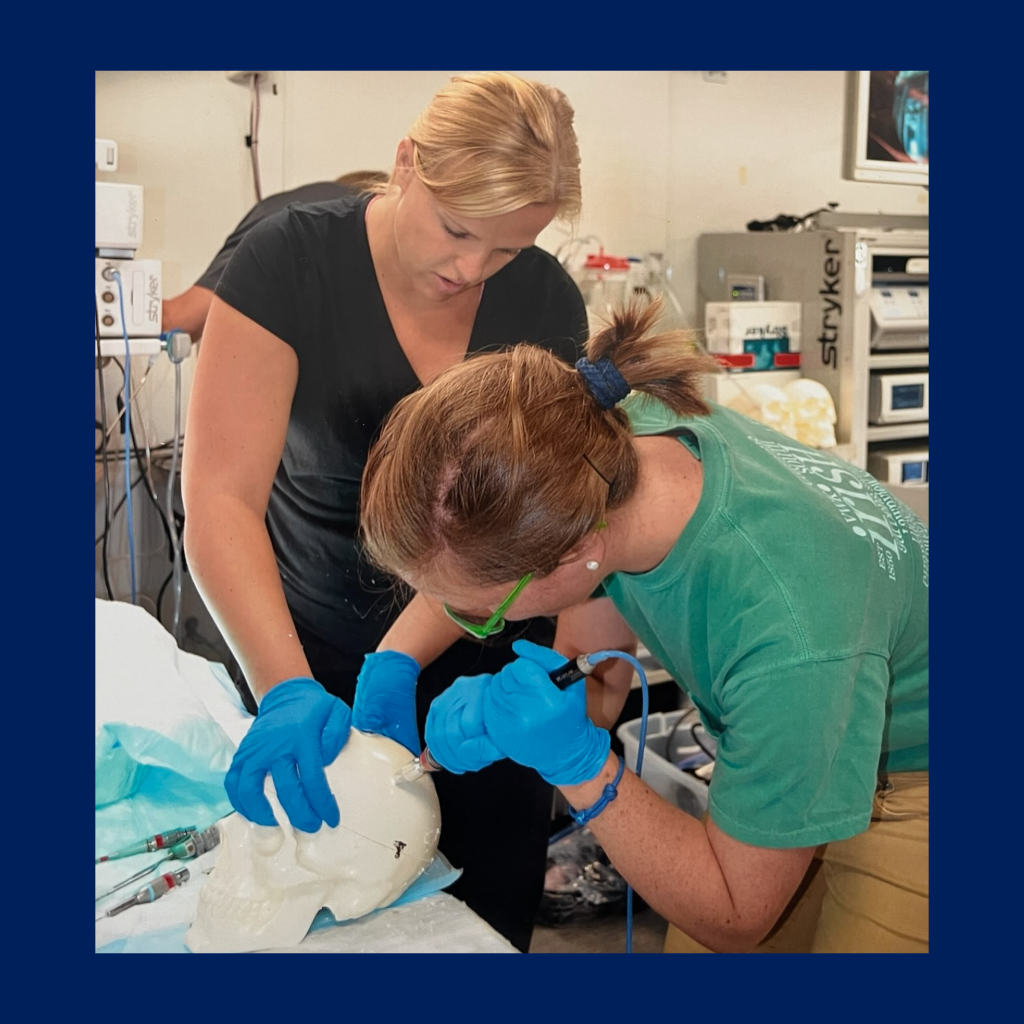
- Course Title – Med Device Design
- 4-credit hour course including lectures, workshops, and student presentations, with weekly team meetings with directors for project progress.
- Builds on clinical observation, guiding students through the identification and development of unmet clinical needs.
- Incorporates brainstorming and prototyping techniques to encourage the creation of potential concepts.
- Utilizes tools like Six Sigma to inform design decisions and clarify design requirements and product specifications.
- The curriculum covers critical topics such as Stakeholder Analysis, Voice of the Customer Methods, and Market Analysis.
- The course has key milestones including Needs Review (October), Design Review (Early November) Concept Review (December)


BME 431
- Course Title – FDA & Intellectual Property
- 2 credits – only offered in the Fall and must be taken before BME 432
- Focuses on IP, patenting, medical innovation pathways
- Teaches FDA regulation terminology and processes
- Covers Regulatory Affairs vs. Regulatory Science
- Emphasizes understanding of prior art, regulatory barriers
- Guides early project planning for commercialization
- Highlights evaluating and protecting intellectual property
- Facilitates navigation of patenting processes
BME 495
- Course Title – Masters Research in BME
- 4-credit course – Continuation of BME 535
- Medical Device Design Course
- Prototyping and commercialization curriculum
- Human Factors Review (February), Design Review (March) Design Day (Early May), culminating with the Exit Exams (Mid May)


BME 438
- Course Title: Introduction to Quality Engineering
- 2-credits – only offered in the spring semester
- Taught by CMTI Academic Director, Dr. Amy Lerner
BME 432
- Course Title – FDA & IP Commercialization
- 2-credit course – only offered in the spring semester
- Focuses on IP and regulatory pathways.
Teaches IP protection, evaluation, optimization - Builds on BME431 material
- Combines lectures, case studies, assignments
- Alternates IP and regulatory topics
- Tailors assignments to student’s interests
- Offers partnership opportunity with FDA
OPT 481
-
Course Title – Gen. Management of New Venture
-
4-credit course – only offered in the spring semester
-
Analyze entrepreneurship from start-up and corporate perspectives
-
Understand skills needed for success in diverse ventures
-
Discuss organization, market opportunities, and financial planning
-
Explore capitalization, fund sourcing, and the due-diligence process
-
Master venture valuation and management
-
Engage in lectures, case studies, and guest talks
Elective Curriculum (12 credits)
Two BME Intensive or Approved Engineering Courses
- 8-Credits
One Graduate Biology Course
- 4-Credits
Additional Requirements
Final Exit Exam
Teaching Assistantship
A student must pass a comprehensive oral examination. This examination is based on the design experience and coursework throughout the year. A written document is submitted, followed by an oral presentation and questions from an examining committee. In addition to the design and commercialization coursework, questions will be drawn from courses satisfying the BME Intensive, Approved Biology and Approved Engineering requirements.
All CMTI MS students receiving tuition-based scholarships are required to serve as Teaching Assistants (TA). CMTI MS students often complete this requirement by serving as a Teaching Assistant for the BME Senior Design course within the undergraduate BME program. This assignment offers an opportunity for MS students to gain experience in project management and supervision, while offering exposure to the product development process in a wider variety of devices. The program and TA experience is spread over two semesters, thus balancing with other coursework over the course of the year. In some circumstances, CMTI MS students may be asked to serve as a TA in other BME courses.

BME Graduate Handbook
Browse Plan B MS structure and list of courses

Senior Design at UR
CMTI students often TA for the BME senior design course

Medical Device Project
Check out past CMTI team projects